10 Grams Of Salt Is Dissolved Ina 100g Of Water
When 10 grams of salt dissolve in 25 grams of water what is the resulting mass of the mixture. About 40 g You can determine how many grams of sodium chloride commonly known as table salt can be dissolved in 100 g of water at 80C by taking a look at its solubility graph.
How Many Grams Of Nacl To Dissolve In 100 G Of H2o Before Attaining Saturation Point Quora
The expression for the molarity of the solution is M o l a l i t y m molar mass of salt mass of salt mass of water 1 Substituting values in the above expression we get m 3 0 3 2 5 0 1 1 0 0 0 0.
10 grams of salt is dissolved ina 100g of water. At 20 C one liter of water can dissolve about 357 grams of salt a concentration of 263. In fact you can expect to be able to dissolve no more than 40 g of sodium chloride per 100 g of water at 80C. A solution contains 100 g of urea in 400 g of water.
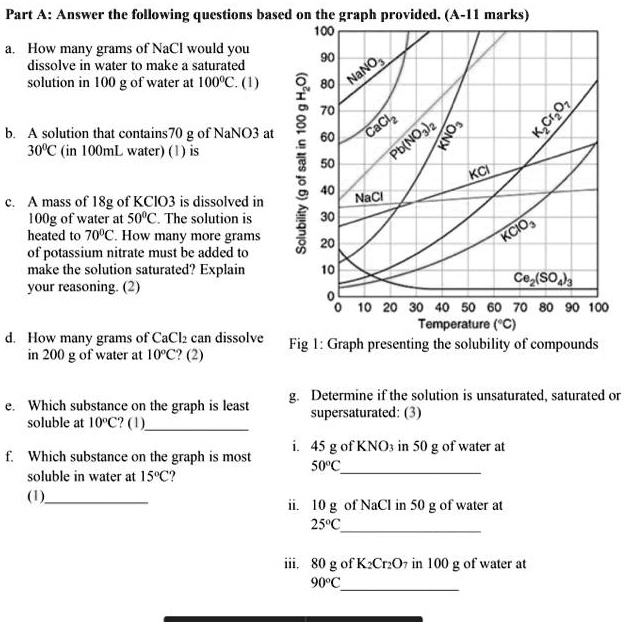
The Graph below provides the solubility of various components at different temperatures as grams of salt dissolved in 100 g of water. Calculate the concentration percentage of the solution by mass. The question says there are 20 g KClO_3 dissolved which means colorred30colorwhitelg -.
A substances solubility graph tells you how its. You require distilled water the salt and a volumetric flask. So at 80C you will ahve an unsaturated solution if you dissolve less than 40 g of sodium chloride and a saturated solution if you dissolve about.
Weigh accurately 10 grams of the salt using an electronic balance. 3 g of a salt of molecular weight 3 0 is dissolved in 2 5 0 g of water. You have 90 grams of water and you add 10 grams of salt sodium chloride.
To find how many grams of salt will gives us a saturated solution in 100 grams of water at the same temperature we can use the rule of three. So the solubility of the salt is 3516 g100 g of water. Because of the Law of Conservation of Mass 5g salt dissolved in 100g of water has a mass of 105g.
Answer 1 of 8. In this regard how many grams of salt must be dissolved in water. You have 250 g of water so the maximum amount of sucrose that will dissolve is.
Working it a little this gives us. Calculate the concentration in terms of mass by mass percentage of the solution. Grams of water over a temperature range of 40C in 10-degree intervals.
Answer 1 of 10. 100 mL of water has a mass of 100 g. A solution contains 40 grams of common salt dissolved in 345 grams the solution.
How many grams of sodium nitrate can be dissolved in 100 grams of water at 10c. 10 mass mass percent concentration. A certain salt is known to have a solubility in water of 252g100g water at 100C and 114g100g water at 20C.
90 grams 10 grams 100 grams mass of total solution. C g 100 g water g 100 g water 10 21 81 20 32 88 30 45 96 What is the effect. This mixes the salt and water faster and makes the salt dissolve faster.
What Temperature Does salt dissolve in water. At boiling 100 C the amount that can be dissolved in one liter of water increases to about 391 grams a concentration of 281. 1 Expert Answer The solubility of sucrose is 179 g per 100 mL of water at 20C.
Producing a graph to represent solubility data Potassium nitrate KNO3 Sodium nitrate NaNO3 Temp. Add the salt to 50 ml of water in a 100ml volumetric flask and swirl until all the salt has. How much salt will crystallize.
At 15C 35g will dissolve therefore 70g-35g 35g will precipitate out. We know that 320 grams of salt in 910 grams of water gives us a saturated solution at 25C. The salt is dissolved in 240 g of water at 100C until no more solid will dissolve.
Key Concepts Dissolving a solid in a liquid depends on the inter. A16 B32 C48 D64 11A student determined the mass in grams of compound X that would saturate 30. As temperature increases its solubility increases as well.
How many grams of sodium nitrate will dissolve in 100g of water at 20 C. The water is the solvent sodium chloride is the solute and the solution is salt water. 10 grams 100 grams 01 --.
240 220 200 Potassium Nitrate bo 180 160 Solubility in g per 100 g of water 140 Sodium Nitrate 120 Nickel Chloride 100 80 60 Sodium Chloride 40 20 0 0 10 Potassium. The Graph below provides the solubility of various components at different temperatures as grams of salt dissolved in 100 g of water. 4 The molality of the solution is 0.
The resulting mass would be 35 grams. Notice however that it does not increase significantly. 300 280 260 Potassium Iodide 240 220 200 Potassium Nitrate 180 160 Solubility in g per 100 g of water 140 Sodium Nitrate 120 Nickel Chloride 100 80 60 Sodium Chloride 40 20 0 Potassium Chlorate 60 70 80.
How many grams of sugar can dissolve in 100 grams of water heated 20 C. 10 more grams of KClO_3 can dissolve. If you add 10g of salt to 100g of.
425 2446 Views. 80 g Hear this out loudPauseBased on the literature at 10C 10 C 80 g of sodium nitrate can be dissolved in 100 g of water. 10 Grams of Salt 056.
43 Votes At 90C 70g will dissolve in 100g of water. 10How many grams of KCl must be dissolved in 200 grams of water to make a saturated solution at 60C. Dissolving and Back Again ObjectiveStudents will be able to develop and explaina particle-level model to describe their observations of water dissolving salt the water evaporating and the salt crystals re-forming.
The curve for KClO_3 is the brown line and according to the 70 oC vertical line a saturated solution of KClO_3 contains colorred30colorwhitelg in 100 g H_2O. To solve this lets look at a solubility curve graph. 300 280 260 Potassium Iodide.
The results are tabulated below.

Quick Answer How Much Is 10 Grams Of Salt Bikehike
Komentar
Posting Komentar